The anti-TNFR2 antibody BI-1808 is part of BioInvent’s tumor-associated regulatory T cells (Treg)-targeting program. TNFR2 is particularly upregulated on Tregs of the tumor microenvironment and has been shown to be important for tumor expansion and survival, representing a new and promising target for cancer immunotherapy.
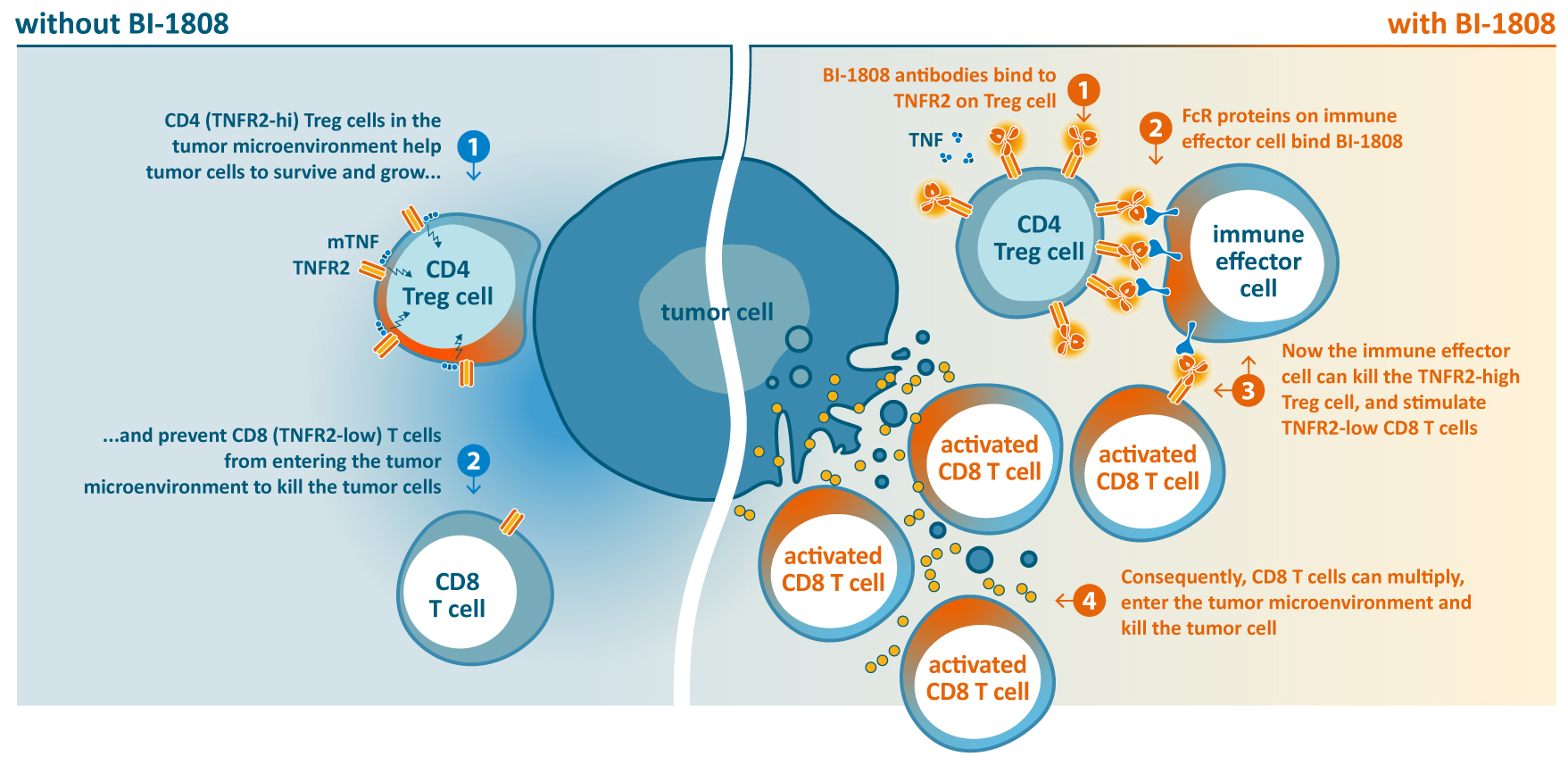
BI-1808 could represent a new class of immunomodulatory agent with the potential to improve efficacy of cancer therapy
STATUS
Efficacy in clinical Phase 1/2a study (NCT04752826) in CTCL
In June 2025, updated positive data from the ongoing Phase 2a dose expansion study of BI-1808 monotherapy in cutaneous T-cell lymphoma (CTCL) was announced. The data was presented at the
European Hematology Association (EHA) 2025 congress. Data showed a 100% disease control rate in nine evaluable patients with CTCL. Forty-five percent of these patients achieved an objective response, with one patient achieving a complete response (CR), three achieving a partial response (PR), and five exhibiting stable disease (SD). Additionally, two patients with peripheral T-cell lymphoma (PTCL) were evaluable, of which one showed a PR, while the other showed SD.
Overall, BI-1808 monotherapy demonstrates promising clinical activity and robust immune engagement. Additionally, BI-1808 was well tolerated, with all treatment-related adverse events reported as mild or moderate (Grade 1-2). Notably, no Grade 3 or higher adverse events were observed. The safety and preliminary efficacy of BI-1808 monotherapy are currently being evaluated in a sub-cohort (Part A) of the ongoing Phase 2a study in patients with T-cell lymphomas, including CTCL. The CTCL monotherapy cohort will be finalized during H2 2025. Subsequently, the Phase 2a evaluation of BI-1808 in combination with pembrolizumab for the treatment of CTCL will be started. These studies will form the basis for the selection of monotherapy or combination to the subsequent pivotal Phase 2 study.
In April 2025, BioInvent received Fast Track Designation from the U.S. Food and Drug Administration (FDA) for BI-1808 for the treatment of CTCL and in March 2025, Orphan Drug Designation was received from the same agency for BI-1808 in T-cell lymphoma (TCL).
T-cell lymphomas include a number of subtypes of T cell-derived non-Hodgkins’s lymphoma, including CTCL. CTCL is a rare and aggressive form that originates in T-lymphocytes residing in the skin. It typically manifests with persistent skin lesions, itching, and potential systemic complications, significantly impacting patients’ quality of life. Each year, approximately 3,000 new cases are diagnosed in the United States with limited effective treatment options available.
STUDY DESIGN
During the first part of the Phase 1/2a study the safety, tolerability, and potential signs of efficacy of BI-1808 as a single agent (part A) and in combination with the anti-PD-1 therapy pembrolizumab (part B) are evaluated in patients with advanced solid tumors and T-cell lymphoma.
The efficacy of BI-1808 as single agent is currently explored in the Phase 2a part of the trial in a larger sample of patients. Expansion cohorts include ovarian cancer, all tumor types and T-cell lymphomas (including CTCL). The dose escalation in Phase 1 Part B has been completed and the Phase 2a dose expansion study for the combination is ongoing. The expansion cohorts include ovarian cancer, all tumor types and T-cell lymphoma (including CTCL).
Read more about the ongoing Phase 1/2a study KEYNOTE-D20
Evaluation of BI-1808 for the treatment of solid tumors and T-cell lymphomas.
OUT-LICENSING AND PARTNERING
Since August 2021, BioInvent has a clinical trial collaboration and supply agreement with MSD, a tradename of Merck & Co., Inc., Rahway, NJ., USA, to evaluate the combination of BI-1808 and MSD’s anti-PD-1 therapy, KEYTRUDA (pembrolizumab).


